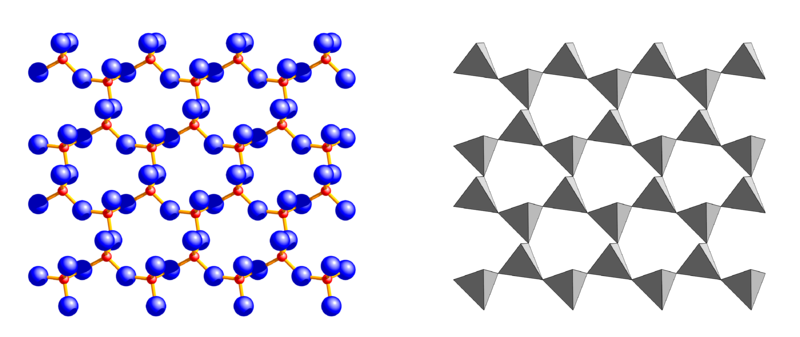
Rather than forming single or double chains as in Pyroxenes and Amphiboles, sometimes the silica tetrahedra are organized into layers, which gives the crystals a lamellar appearance, as in the famous “micas.” But in this case the layers of silica tetrahedra can be coupled with layers that contain aluminum instead of silicon. Aluminum behaves a little differently and orients its bonds according to the vertices of an octahedron, a solid with eight triangular faces (two pyramids joined by the base). The presence of these foils of tetrahedra and octahedra gives these minerals the name Phyllosilicates. The double-latered ones have one layer of tetrahedra and one of octahedra side by side, the three-layered ones have two layers of tetrahedra with one of octahedra in between. One group of phyllosilicates is made of microscopic lamellar crystals, on the order of a micron: these are the clay minerals. Sometimes the layers are rolled on themselves creating filaments as in the case of asbestos and talc.
Deep in the crust, where pressure and temperature are considerably high, the silicates composing the rocks are transformed in different minerals that are stable in such extreme conditions. Often, the flat or elongated shape of those minerals suggests they underwent very high pressure. When geological processes cause pressure and temperature to go back to lower values, it may happen that some of the minerals become unstable again and recover the forms which are typical of the new situation – or they melt to form new magma.
A very important group of silicates, typically seen in flat-shaped thin leafs, is called Phyllosilicates, literally “leaf – or sheet silicates” (top).
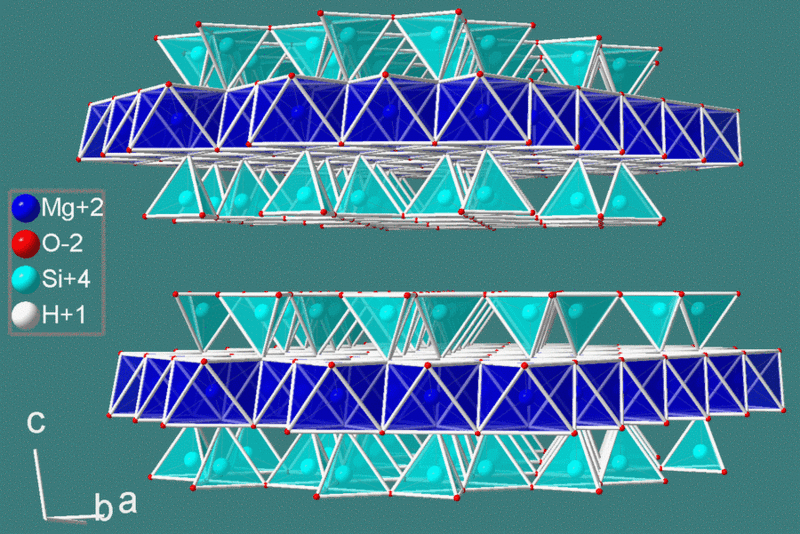
Silica tetrahedron sheets (T) with the formula Si2O52- are superimposed to octahedron sheets (O) that can be of two different types: the Mg3(OH)6 Brucite type or the Al2(OH)6 Gibbsite type. With high magnesium content, combinations of silica tetrahedron sheets and brucite octahedron sheets (T-O) form the phyllosilicate called Serpentine (T:Si2O5 + O:Mg3(OH)6), a mineral known for the fibrous aspect of its Crysotil variety (asbestos); filaments are formed by T-O sheets rolled up on themselves. Adding another tetrahedron T sheet when silica is abundant, generates a sandwich T-O-T structure (below) typical of the mineral known as Talc.
If the aluminum is the abundant one, the T-O packing of silica tetrahedrons and Gibbsite octahedron gives birth to Kaolinite, a typically white clay mineral (see below); the corresponding T-O-T packing generates Pyrophyllite.
Adding fluorine, potassium and aluminum in high pressure and temperature conditions can lead to the formation of Mica: the Flogopite variety is found in magnesium-rich environments (if iron is also present we have Biotite, the black Mica); Muscovite (white Mica) generates when aluminum is abundant but fluorine lacks. The Micas are those typically sheeted minerals showing an easy basal cleavage.
Clay minerals
Clay minerals are phyllosilicates that form lamellar crystals of the order of microns in size.
Kaolinite is the aluminum clay mineral and has a double-layered, T-O structure. It is used as an inert material (kaolin) in the paper industry as a filler between fibers (especially in coated paper) and in construction in the preparation of plasters.
Montmorillonite (alumino-magnesian) and Illite (potassic, chemically similar to Muscovite mica) are three-layered phyllosilicates (T-O-T). Montomorillonite, like, to a lesser extent, Illite, is the main component of drilling muds (bentonite); illite, whose name comes from Illinois, USA, where it was discovered, is the only clay that does not expand and has some food uses.
Chlorite (or rather the chlorite group, which contains about a dozen minerals) contains iron and magnesium and has a four-layered structure (T-O-T-O).
Clay minerals have the property of absorbing fluids; Montmorillonite is the one that contains the most water in the molecule. They are mainly formed by the weathering of other silicates and during metamorphism generate the micas that most closely match their chemism.
Translated with DeepL.com (free version)