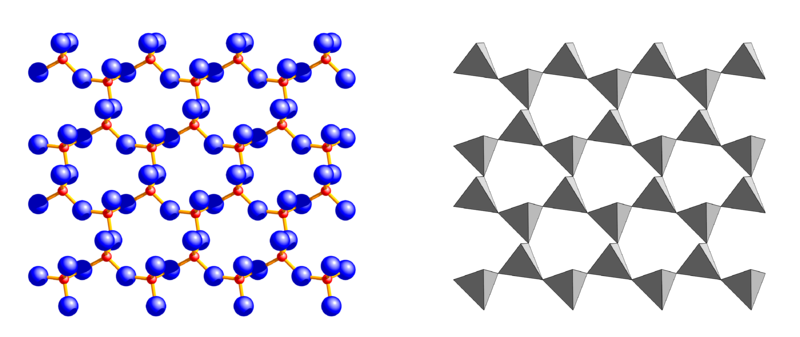
Deep in the crust, where pressure and temperature are considerably high, the silicates composing the rocks are transformed in different minerals that are stable in such extreme conditions. Often, the flat or elongated shape of those minerals suggests they underwent very high pressure. When geological processes cause pressure and temperature to go back to lower values, it may happen that some of the minerals become unstable again and recover the forms which are typical of the new situation – or they melt to form new magma.
A very important group of silicates, typically seen in flat-shaped thin leafs, is called Phyllosilicates, literally “leaf – or sheet silicates” (top).
Silica tetrahedron sheets (T) with the formula Si2O52- are superimposed to octahedron sheets (O) that can be of two different types: the Mg3(OH)6 Brucite type or the Al2(OH)6 Gibbsite type. With high magnesium content, combinations of silica tetrahedron sheets and brucite octahedron sheets (T-O) form the phyllosilicate called Serpentine (T:Si2O5 + O:Mg3(OH)6), a mineral known for the fibrous aspect of its Crysotil variety (asbestos); filaments are formed by T-O sheets rolled up on themselves. Adding another tetrahedron T sheet when silica is abundant, generates a sandwich T-O-T structure (below) typical of the mineral known as Talc.
If the aluminum is the abundant one, the T-O packing of silica tetrahedrons and Gibbsite octahedron gives birth to Kaolinite, a typically white clay mineral; the corresponding T-O-T packing generates Pyrophyllite.
Adding fluorine, potassium and aluminum in high pressure and temperature conditions can lead to the formation of Mica: the Flogopite variety is found in magnesium-rich environments (if iron is also present we have Biotite, the black Mica); Muscovite (white Mica) generates when aluminum is abundant but fluorine lacks. The Micas are those typically sheeted minerals showing an easy basal cleavage.
Another typical metamorphic mineral family are the Garnets. They are Nesosilicates, that is they are structured by isolated groups of SiO44- tetrahedrons; in this case the groups are made of 3 tetrahedrons: (SiO4)35-. Aluminum is often found bonded to those groups to form the typical Garnet radical: Al2(SiO4)33-, but ferric iron (III) is also common in Andradite, as chrome is in Uvarovite. This radical may also bond magnesium, iron and calcium.
|
|
||||||||||
| Mg | Fe | Mn | Fe3+ | Al | Cr | |||||
 |
 |
 |
 |
 |
 |
|||||
| Mg3Al2(SiO4)3 | Fe3Al2(SiO4)3 | Mn3Al2(SiO4)3 | Ca3Fe2(SiO4)3 | Ca3Al2(SiO4)3 | Ca3Cr2(SiO4)3 | |||||
| Pirope | Almandine | Spessartite | Andradite | Grossular | Uvarovite | |||||
| <——— | Calcic Garnets | ———> | ||||||||
|
|
||||||||||
Another group of nesosilicates is an important indicator of a metamorphic rock’s formation temperature: the aluminum silicate Al2SiO5. It exists in three polymorphs, never to be found together in the same rock, since they are stable at different pressure and temperature conditions: Andalusite forms at low pressure (LP) but variable temperatures; Cyanite only forms at high pressure (HP > 4 Kbar); Syllimanite forms at high temperature (HT) but variable pressure.
| HP | LP | HT |
 |
 |
 |
| Al2SiO5 | ||
| Cyanite | Andalusite | Syllimanite |
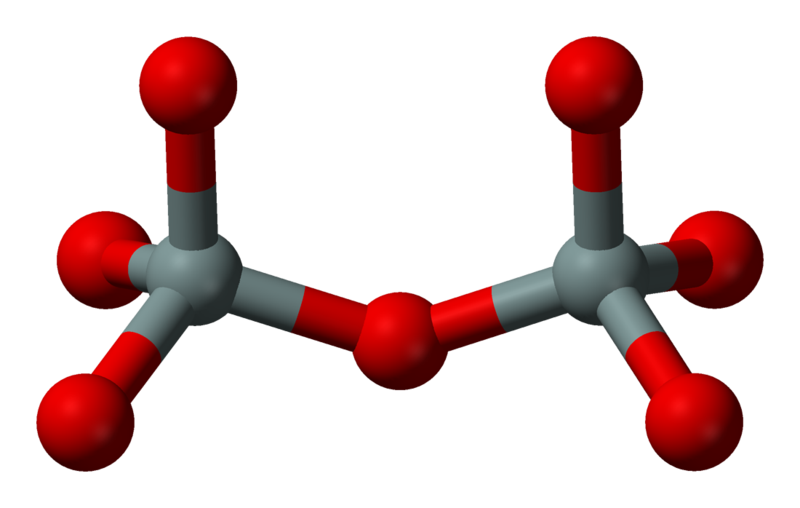 In some cases, the tetrahedron groups are formed by Si2O76- pairs (left) and are called Sorosilicates. In the Epidote group the tetrahedrons are bonded to calcium, ferric iron and aluminum, and also to a SiO4 group. Most common Epidotes are the Zoisite (orthorombic) and the Clinozoisite (monocline):
In some cases, the tetrahedron groups are formed by Si2O76- pairs (left) and are called Sorosilicates. In the Epidote group the tetrahedrons are bonded to calcium, ferric iron and aluminum, and also to a SiO4 group. Most common Epidotes are the Zoisite (orthorombic) and the Clinozoisite (monocline):
|
|
|||
| Fe3+ | Al | ||
 |
 |
||
| Ca2(Al,Fe)3O(SiO4)(Si2O7)(OH) | Ca2Al3O(SiO4)(Si2O7)(OH) | ||
| Epidote | Zoisite | ||
|
|
|||